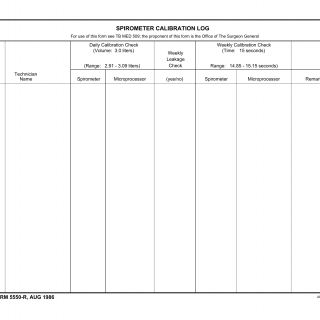
DA Form 5550-R. Spirometer Calibration Log (LRA)
DA Form 5550-R, titled Spirometer Calibration Log, is used to maintain a record of calibration activities for spirometers. This form serves as a log to track calibration dates, personnel responsible for calibration, and any remarks related to spirometer calibration.
The form consists of sections where calibration-related information is documented, including the calibration date, spirometer model, serial number, and the name of the individual conducting the calibration. It includes spaces to record any remarks or notes regarding the calibration process.
Key fields in this form include calibration details such as the date, spirometer information, and calibration personnel. Accurate completion of this form is essential to ensure that spirometers are properly calibrated and functioning accurately.
Application Example: A medical facility that uses spirometers for lung function testing would use DA Form 5550-R to log calibration activities. By accurately completing the form and documenting calibration details, the facility maintains the accuracy and reliability of spirometry results.
No additional documents are explicitly mentioned as required for filling this form.
Related Form: DA Form 5551-R is a related form used for documenting spirometry flow measurements.